High-quality, Accurate Testing Services
Initiatives for quality control
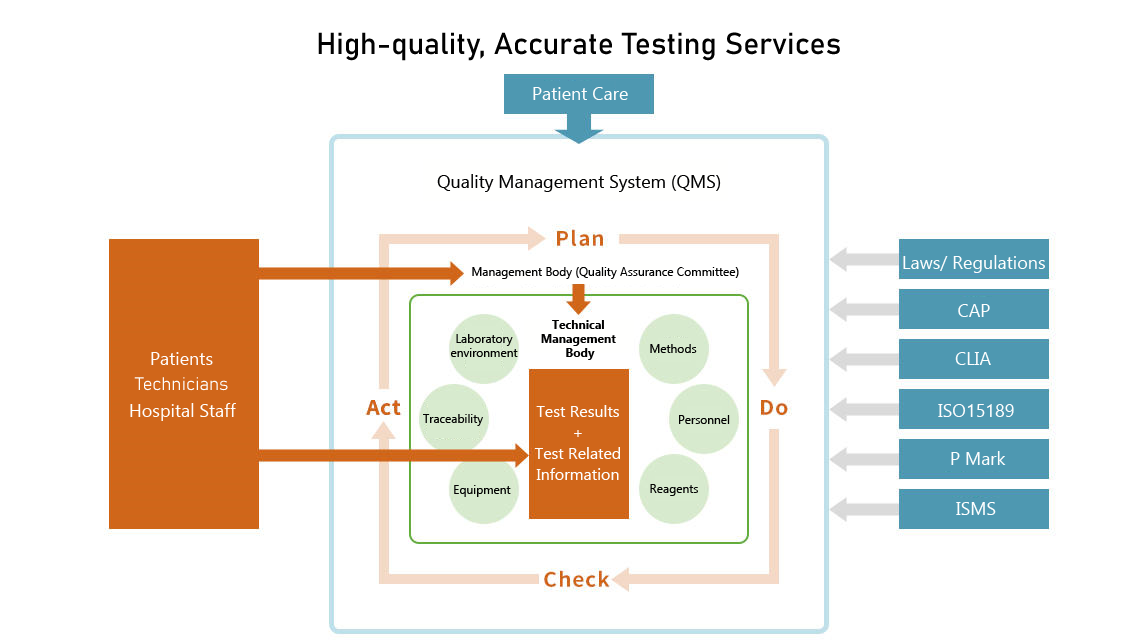
SRL is constantly striving to achieve the highest standards in test quality, and has developed a quality management system that is always listening to customer feedback and puts patient care first.
By proactively undergoing external quality assessments for management and operations, we have established a quality system that is both effective and objective.
Our Commitment to Accuracy
At SRL, we are continuously working to improve accuracy, and have introduced a quality control system to ensure the reliability of measurement values in our clinical testing.
To ensure samples are sent to us with confidence, our system must not only focus on ensuring the accurate measurement of the test samples, but should be present from when the sample enters our care right through to providing the results, and throughout all of our testing services. We believe this can provide customers with an even more reliable testing service.
It is not only our Hachioji Laboratory complex, but all our laboratories throughout Japan that proactively undergo external assessments and strive for ongoing improvement.
Since first acquiring the international standard ISO 15189 for medical laboratories in 2003, the Hachioji Laboratory complex has been developing a quality assurance system in line with the standards for quality and competence.
After being certified in 2005 by the Japan Accreditation Board, they have been continuously working towards the highest standards of quality.

ISO15189 Certified Laboratories
- Hachioji / MUQS Laboratory
December 2005
Obtained ISO15189 Medical Laboratories certification from the Japan Accreditation Board (JAB)

- Sagamihara Laboratory
March 2008
Obtained ISO15189 Medical Laboratories certification from the Japan Accreditation Board (JAB)
SRL's main laboratory, the Hachioji Laboratory complex, was its first to be CAP (Collage of American Pathologists) accredited by a recognized accreditation agency.

CAP Accredited Laboratory
- SRL Inc Main Laboratory- Central laboratory
- MSRL Inc MUQS Laboratory- MUQS laboratory- Hachioji Laboratory- Hachioji No.3 Laboratory
- SRL Inc Sagamihara Laboratory

CLIA Certified Laboratory
- MUQS Laboratory
Certified in October 2015 by the American Clinical Laboratory Improvement Amendments (CLIA) of 1988 operated by Centers for Medicare and Medicaid Services (CMS).

PrivacyMark
We were the first commercial clinical laboratory to be awarded the “PrivacyMark” from the Japan Information Processing Development Corporation (JIPDEC) for measures taken to protect the personal information of patients.

